Products

 Explore
Explore

Lithium Polymer Battery
1550mAh lithium polymer battery 3.7V 3.8V 7.4V 11.1V 503480
1450mAh lithium polymer battery 3.7V 3.8V 7.4V 11.1V 553470
1400mAh lithium polymer battery 3.7V 3.8V 7.4V 11.1V 383498
1350mAh lithium polymer battery 3.7V 3.8V 7.4V 11.1V 404762
1300mAh lithium polymer battery 3.7V 3.8V 7.4V 11.1V 103636
1250mAh lithium polymer battery 3.7V 3.8V 7.4V 11.1V 363392

Energy Storage Battery
Energy Storage Solar battery LiFePO4 24V 100AH 5kWh for UPS EPS Power Systems
Rack Mounted LiFePO4 battery 48V 100AH 5kWh for ESS EBSS Energy Storage Systems
Rack mounted energy storage battery 25.6V 200Ah for industry business resident solar power
Cabinet case rack mounted lifepo4 battery 51.2V 100Ah 5kWh for solar energy storage systems
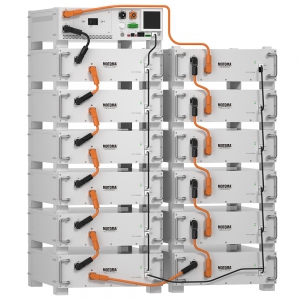
Rack Stacked Lithium LiFePO4 Battery Pack 614.4V 150Ah for Business Uninterruptable Power System
Stackable Lithium Battery LiFePO4 614.4V 100Ah for Commercial UPS EPS Power Solutions
1550mAh lithium polymer battery 3.7V 3.8V 7.4V 11.1V 503480
Nominal Voltage: 3.7 V
Nominal Capacity: 1550 mAh
High battery consistency
Low internal resistance
Volume energy density can reach 500Wh/L – 620Wh/L
Excellent safety performance
Small and light-weight
Factory-direct price
UL, IEC62133, UN38.3, CB, CQC, PS...
1450mAh lithium polymer battery 3.7V 3.8V 7.4V 11.1V 553470
Nominal Voltage: 3.7 V
Nominal Capacity: 1450 mAh
High battery consistency
Low internal resistance
Volume energy density can reach 500Wh/L – 620Wh/L
Excellent safety performance
Small and light-weight
Factory-direct price
UL, IEC62133, UN38.3, CB, CQC, PS...
1400mAh lithium polymer battery 3.7V 3.8V 7.4V 11.1V 383498
Nominal Voltage: 3.7 V
Nominal Capacity: 1400 mAh
High battery consistency
Low internal resistance
Volume energy density can reach 500Wh/L – 620Wh/L
Excellent safety performance
Small and light-weight
Factory-direct price
UL, IEC62133, UN38.3, CB, CQC, PS...
1350mAh lithium polymer battery 3.7V 3.8V 7.4V 11.1V 404762
Nominal Voltage: 3.7 V
Nominal Capacity: 1350 mAh
High battery consistency
Low internal resistance
Volume energy density can reach 500Wh/L – 620Wh/L
Excellent safety performance
Small and light-weight
Factory-direct price
UL, IEC62133, UN38.3, CB, CQC, PS...
1300mAh lithium polymer battery 3.7V 3.8V 7.4V 11.1V 103636
Nominal Voltage: 3.7 V
Nominal Capacity: 1300 mAh
High battery consistency
Low internal resistance
Volume energy density can reach 500Wh/L – 620Wh/L
Excellent safety performance
Small and light-weight
Factory-direct price
UL, IEC62133, UN38.3, CB, CQC, PS...
1250mAh lithium polymer battery 3.7V 3.8V 7.4V 11.1V 363392
Nominal Voltage: 3.7 V
Nominal Capacity: 1250 mAh
High battery consistency
Low internal resistance
Volume energy density can reach 500Wh/L – 620Wh/L
Excellent safety performance
Small and light-weight
Factory-direct price
UL, IEC62133, UN38.3, CB, CQC, PS...
Ultra Thin Lithium Polymer Battery 0.9mm
Ultra-thin design.
High energy density.
Long lifespan.
Excellent safety.
Battery Chemistry: Lithium Ion Polymer
Battery Terminations: PC Pins (Horizontal) / (Vertical), SMT (SMD) Mount, Solder Tabs, Wires, Cables with Connectors.
Battery Customizing: Custom design shap...
LiPo Battery 3.7V Ultra Thin 120mAh
Ultra-thin design.
Nominal Voltage: 3.7 V
Nominal Capacity: 120 mAh
High battery consistency
Low internal resistance
Sufficient battery capacity
Outstanding cycle life
Battery Chemistry: Lithium Ion Poly
Original lipo battery factory from raw material to batter...
3.7V Ultra Thin LiPo Battery 80mAh
Ultra-thin design.
Nominal Voltage: 3.7 V
Nominal Capacity: 80 mAh
Low internal resistance
Sufficient battery capacity
Outstanding cycle life
Optimized PCM design
In series connection or in parallel connection is allowed
Excellent safety.
Battery Chemistry:...
3.7V Fast Charging Lithium Battery 720mAh
Nominal Voltage: 3.7 V
Nominal Capacity: 720 mAh
Working temperature range: -20℃ ~ +60℃
High battery consistency
Low internal resistance
Sufficient battery capacity
Outstanding cycle life
Battery Chemistry: Lithium Polymer
Battery Terminations: PC Pins (Horiz...
3.85V Fast Charging LiPo Battery 110mAh
Nominal Voltage: 3.85 V
Nominal Capacity: 110 mAh
Working temperature range: -20℃ ~ +60℃
Battery Chemistry: Lithium Ion Polymer
High battery consistency
Low internal resistance
Sufficient battery capacity
Outstanding cycle life
Battery Terminations: PC Pins (...
Fast Charging Lithium Polymer Battery 2C 5C
2C~5C fast charging battery.
Charge 70% capacity in very short time.
Long cycle life.
Good consistency, low self-discharge.
None-memory li poly battery.
Excellent safe, environment-friendly.
Battery Chemistry: Lithium Ion Polymer
Battery Terminations: PC Pins (Hori...
3.7V High Density LiPo Battery 1500mAh
Nominal Voltage: 3.7 V
Nominal Capacity: 1500 mAh
Working temperature range: -20℃ ~ +60℃
High battery consistency
Low internal resistance
Sufficient battery capacity
Outstanding cycle life
Battery Chemistry: Lithium Ion Polymer
Battery Terminations: PC Pins (...
11.1V High Density LiPo Battery 2000mAh
Nominal Voltage: 11.1 V
Nominal Capacity: 2000 mAh
Working temperature range: -20℃ ~ +60℃
Sufficient battery capacity
Outstanding cycle life
Optimized PCM design
In series connection or in parallel connection is allowed
Battery Chemistry: Lithium Ion Polymer
...
3.8V High Density LiPo Battery 1300 mAh
Nominal Voltage: 3.8 V
Nominal Capacity: 1300 mAh
Working temperature range: -20℃ ~ +60℃
Excellent safe
Small and light-weight
None-memory battery
Environment friendly battery
High battery consistency
Battery Chemistry: Lithium Ion Polymer
Battery Termina...
3.7V LiPo Battery High Density 7500mAh
Nominal Voltage: 3.7 V
Nominal Capacity: 7500 mAh
Working temperature range: -20℃ ~ +60℃
Excellent safe
Small and light-weight
None-memory battery
Environment friendly battery
Optimized PCM design
In series connection or in parallel connection is allowed
...
3.7V Lithium Polymer Battery High Density 900mAh
Nominal Voltage: 3.7 V
Nominal Capacity: 900 mAh
Working temperature range: -20℃ ~ +60℃
Low internal resistance
Sufficient battery capacity
Outstanding cycle life
Optimized PCM design
In series connection or in parallel connection is allowed
Battery Chemistry...
LiPo Battery High Density 3.7V 6000mAh
Nominal Voltage: 3.7 V
Nominal Capacity: 6000 mAh
Working temperature range: -20℃ ~ +60℃
High battery consistency
Low internal resistance
Sufficient battery capacity
Outstanding cycle life
Battery Chemistry: Lithium Ion Polymer
Battery Terminations: PC Pins (...
Energy Storage Solar battery LiFePO4 24V 100AH 5kWh for UPS EPS Power Systems
Rated Power 25.6 V 200 AH (5.12 KWH)
Made By Fresh Grade A Cells
6000 Cycles @DOD 80%
Intelligent BMS With Advanced Software Improves Performance
Support Parallel Up to 15 Batteries
Max Charging and Discharging Current 150A
Communication: CAN/RS232/RS485
SKU: M64UE L...
Rack Mounted LiFePO4 battery 48V 100AH 5kWh for ESS EBSS Energy Storage Systems
Rated Power 52.6 V 100 AH (5.12 KWH)
Made By Fresh Grade A Cells
6000 Cycles @DOD 80%
Intelligent BMS With Advanced Software Improves Performance
Support Parallel Up to 15 Batteries
Max Charging and Discharging Current 100A
Communication: CAN/RS232/RS485
SKU: M84UE L...
Rack mounted energy storage battery 25.6V 200Ah for industry business resident solar power
Rated Power 25.6 V 200 AH (5.12 KWH)
Made By Fresh Grade A Cells
>6000 Cycles @DOD 80%
Intelligent BMS With Advanced Software Improves Performance
Support Parallel Up to 15 Batteries
Max Charging and Discharging Current 150A
Screen For Real-Time Monitoring
Commu...
Cabinet case rack mounted lifepo4 battery 51.2V 100Ah 5kWh for solar energy storage systems
Rated Power 51.2 V 100 AH (5.12 KWH)
Made By Fresh Grade A Cells
>6000 Cycles @DOD 80%
Intelligent BMS With Advanced Software Improves Performance
Support Parallel Up to 15 Batteries
Max Charging and Discharging Current 150A
Screen For Real-Time Monitoring
Commu...
Rack Stacked Lithium LiFePO4 Battery Pack 614.4V 150Ah for Business Uninterruptable Power System
Rated Power 614.4 V 150 AH (92.16 KWH)
Made By Fresh Grade A Cells
>6000 Cycles @DOD 80%
Intelligent BMS Improves Performance
Support Parallel Up to 10 Batteries
Max Charging and Discharging Current 100A
Screen For Real-Time Monitoring
Communication: CAN/RS485
...
Stackable Lithium Battery LiFePO4 614.4V 100Ah for Commercial UPS EPS Power Solutions
Rated Power 614.4 V 100 AH (61.44 KWH)
Made By Fresh Grade A Cells
6000 Cycles @DOD 80%
Intelligent BMS Improves Performance
Support Parallel Up to 12 Batteries
Max Charging and Discharging Current 100A
Screen For Real-Time Monitoring
Communication: CAN/RS485/LAN
...
Solutions
Solutions